Pharmaceutical Launch
Novartis was launching its first fixed dose combination antihypertensive drug therapy, including its blockbuster valsartan (Diovan) combined with a generic version of Pfizer’s amlodipine (Norvasc).
A key insight from cardiologists in the US led us to understand that the order in which we wrote and presented the drug combination’s generic components affected the efficacy perception of the new drug.
Novartis launched Exforge as amlodipine/valsartan with a clear positioning as the most effective antihypertensive therapy available. Sales results exceeded expectations.
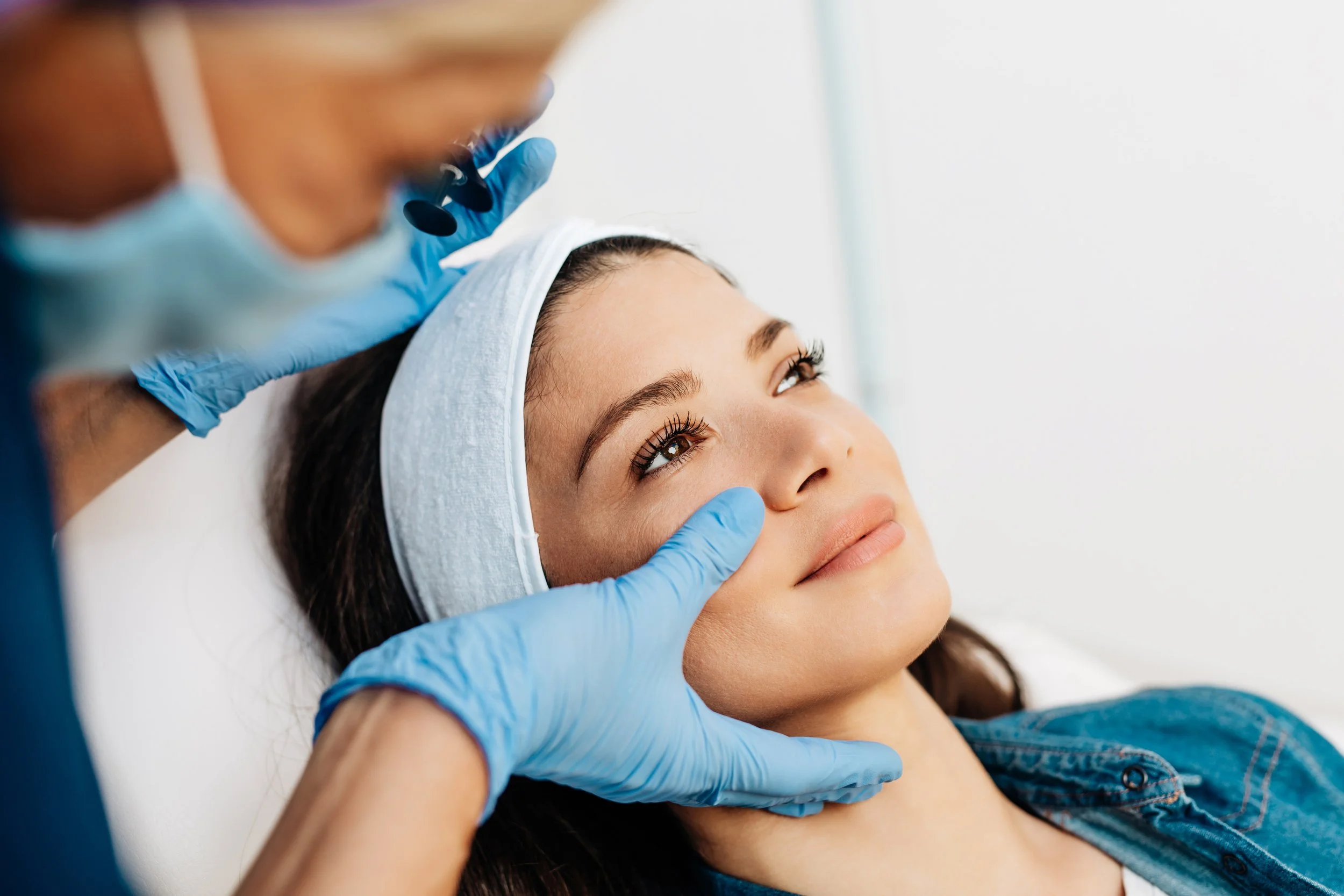
Facial Injectable Relaunch
The facial injectable space was quickly becoming undifferentiated and generisized and brands were losing pricing power.
We relaunched Restylane Skin Boosters, highlighting its unique NASHA technology and the long lasting "hydration from within" benefits it provides, differentiating from the myriad of available mesotherapies.
Focusing on a proprietary technology and highlighting its core benefit allowed Restylane to increase prices and gain market share simultaneously.
Repositioning a Topical Anesthetic
Pliaglis, a topical anesthetic, had a very low share in a dynamic and growing aesthetic market in Italy.
We worked with the marketing team and identified two key messages that would allow us to reposition and promote the brand vs. the market leader:
30 vs. 60 min onset of action
No need for occlusion
As a result, Pliaglis sales grew sixfold within 12 months and kept growing thereafter to overtake the incumbent and become the market leader.